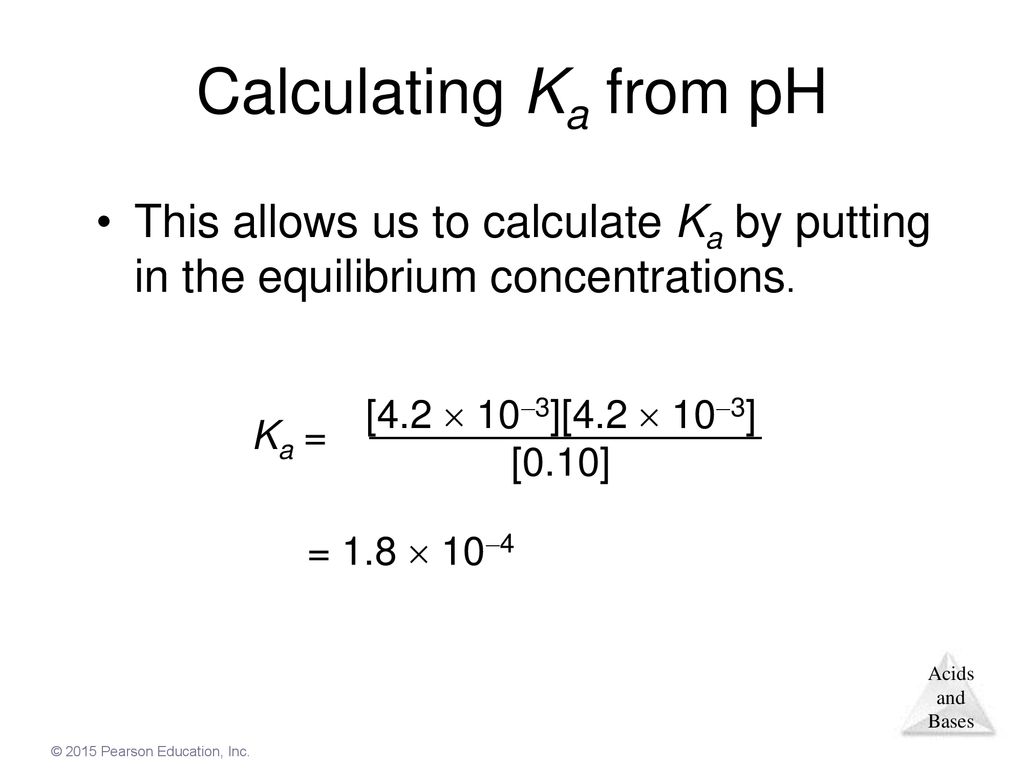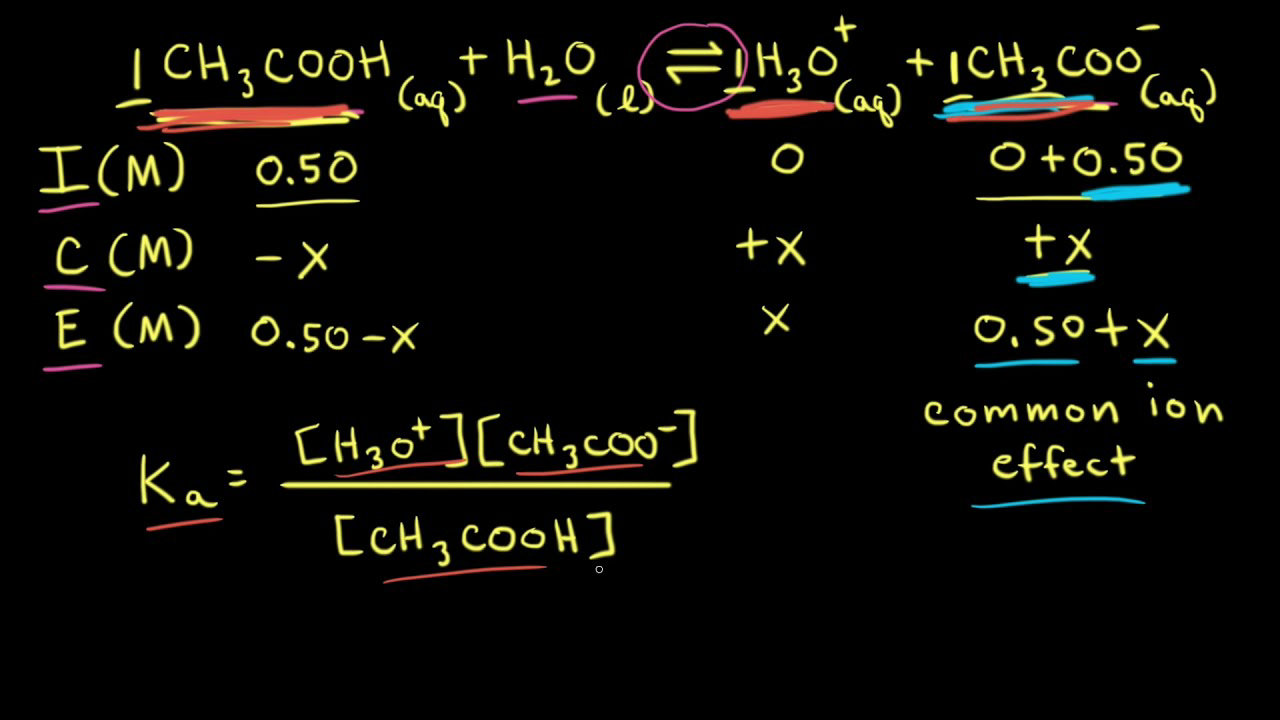
Worked example: Calculating the pH after a weak acid–strong base reaction (excess acid) (video) | Khan Academy

Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer - Video & Lesson Transcript | Study.com

SOLVED: Suppose the pH measure of how acidic or basic a water is) value of a soil in a certain area has the following distribution function: k(z2 20z + 100) , 3 <


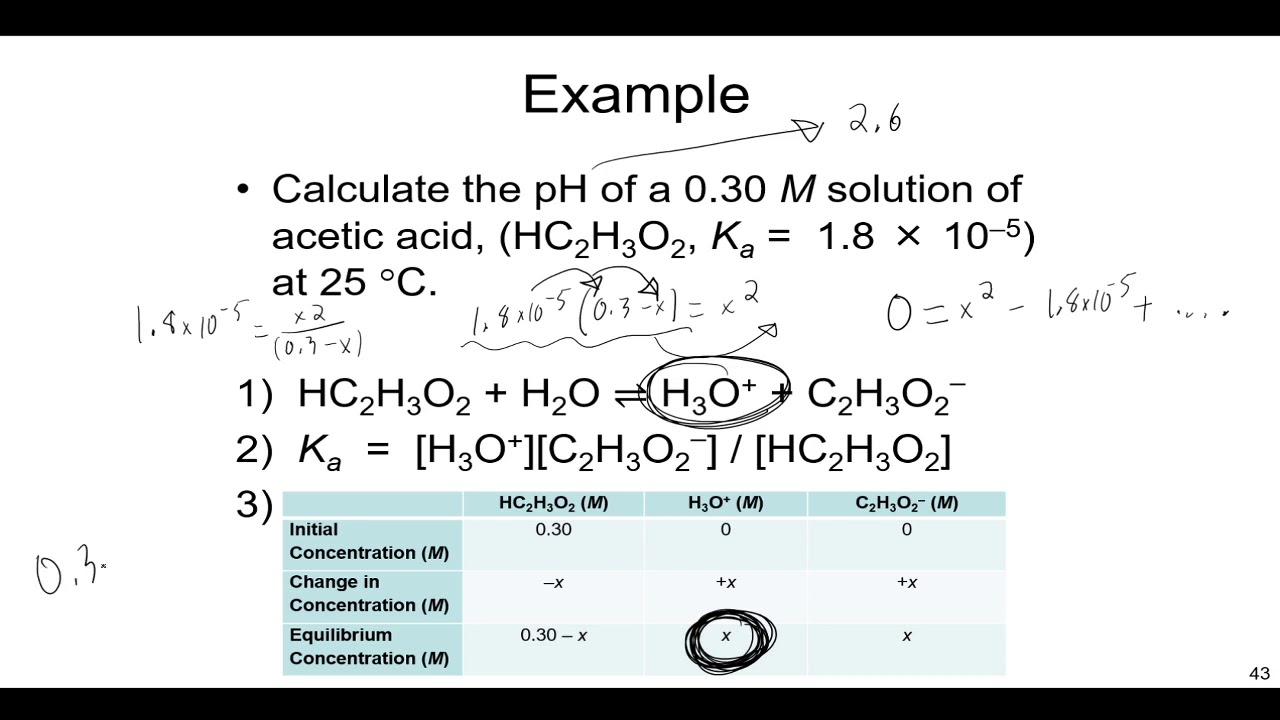
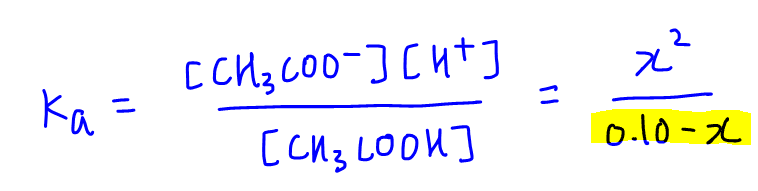
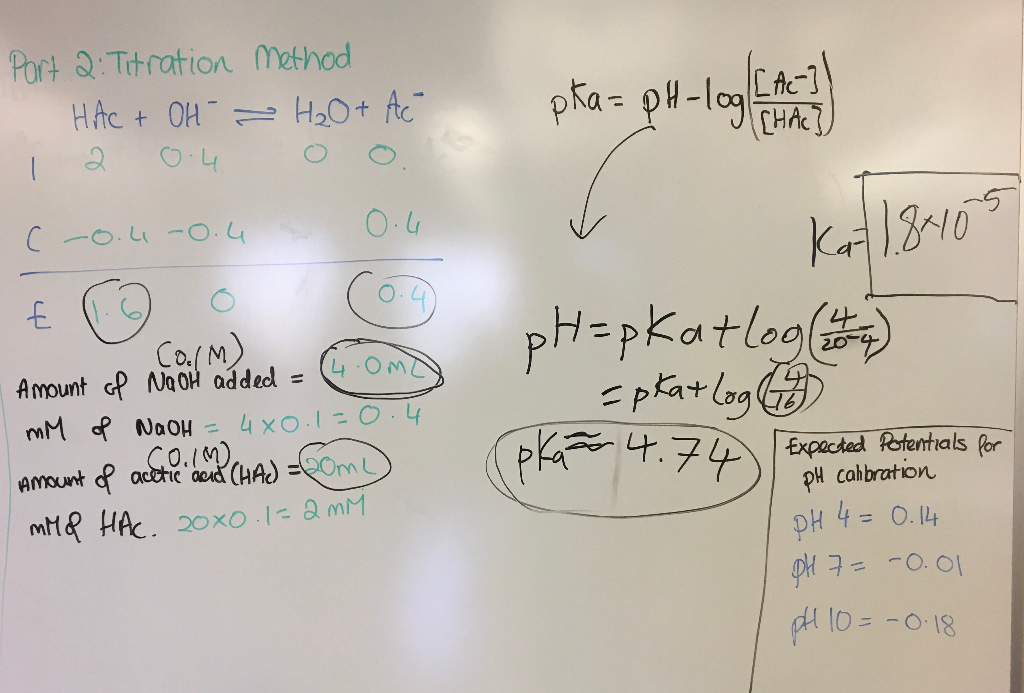

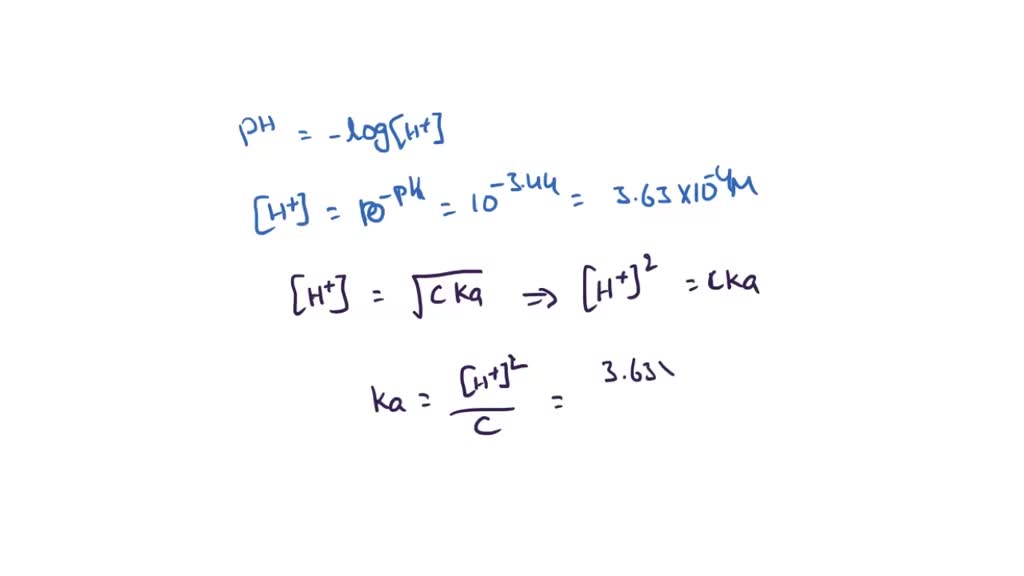

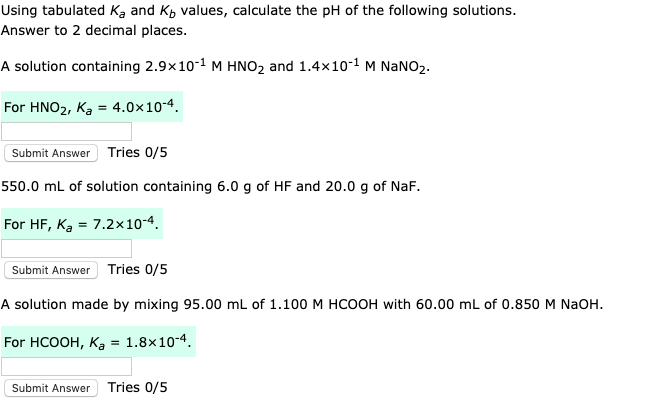
![Calculating [H+] and pH from Ka Calculating [H+] and pH from Ka](https://www.mi.mun.ca/users/pfisher/chemistry1011_134/img013.gif)