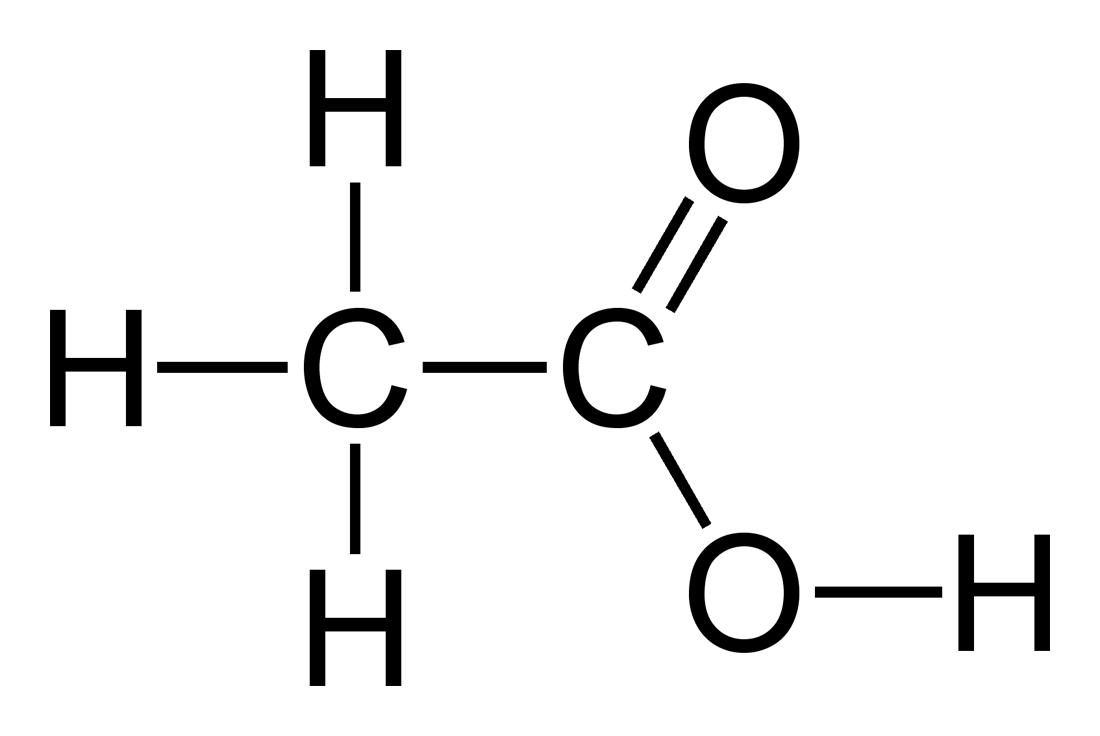
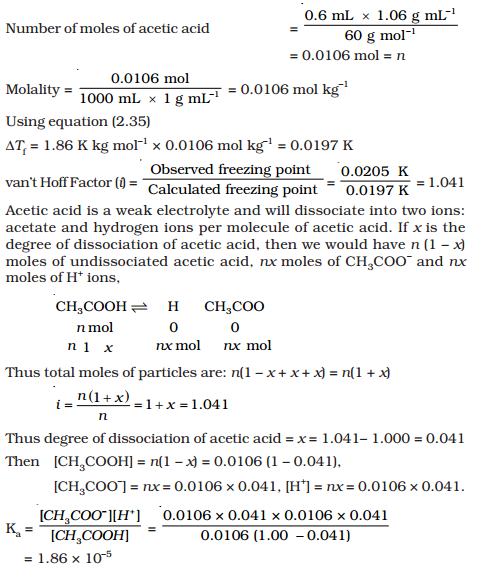
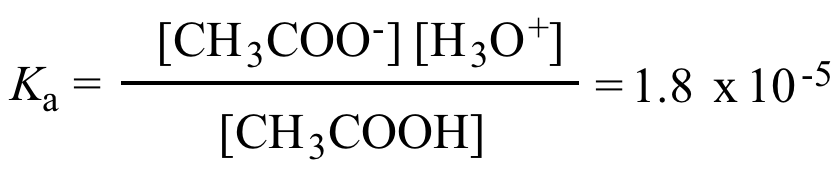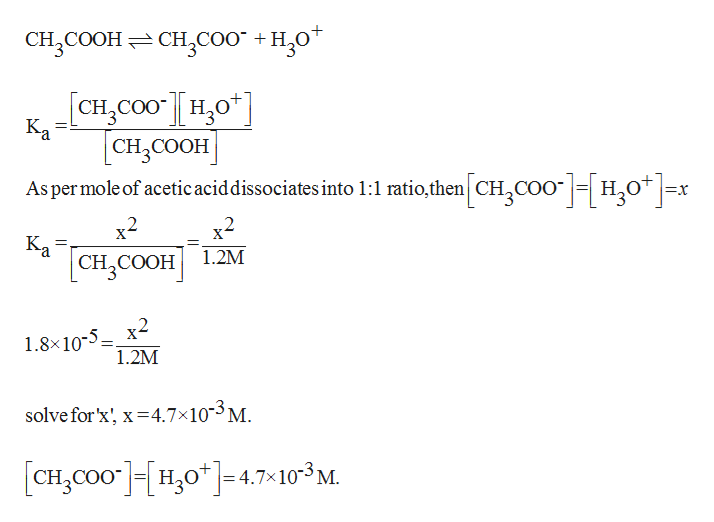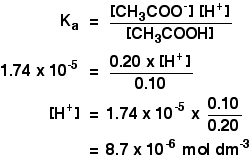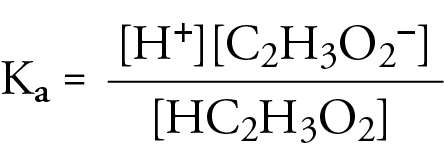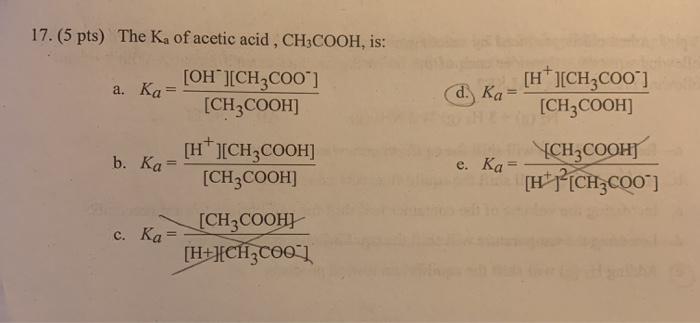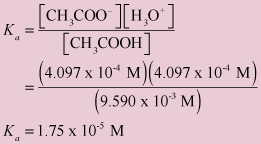A student prepared 0.10M acetic acid solution and experimentally measured its pH to be 2.88. Calculate Ka - Brainly.in
How to calculate the pH of 0.010 molarity acetic acid solution, if its dissociation constant is 1.8*10^-5 - Quora

A 0 05 n sol of acetic acid is found to be 1 9+ ionised at 25 c calculate - Chemistry - Equilibrium - 13276973 | Meritnation.com

Ka for CH3COOH is 1.8×10^-5. find out the % dissociation of 0.2M CH3COOH in 0.1M HCl solution? - EduRev NEET Question

How To Calculate the PH of a Buffer Solution | Equation & Example - Video & Lesson Transcript | Study.com

The ionization constant of acetic acid `1.74xx10^(-5)`. Calculate the degree of dissociation of ... - YouTube
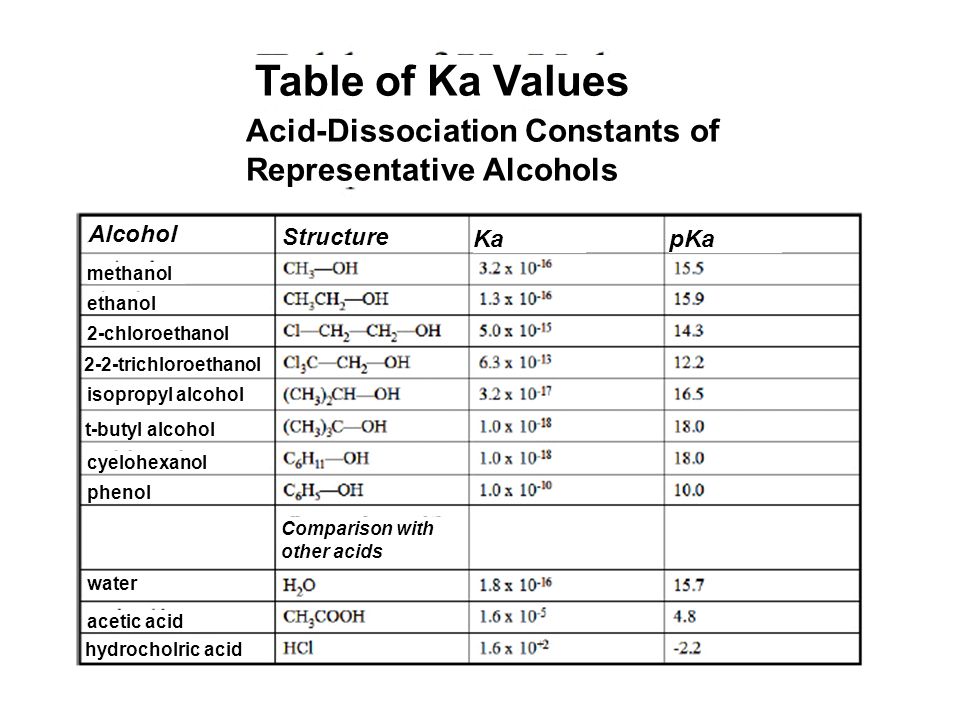
Table of Ka Values Acid-Dissociation Constants of Representative Alcohols Alcohol Structure Ka pKa methanol ethanol 2-chloroethanol 2-2-trichloroethanol. - ppt video online download