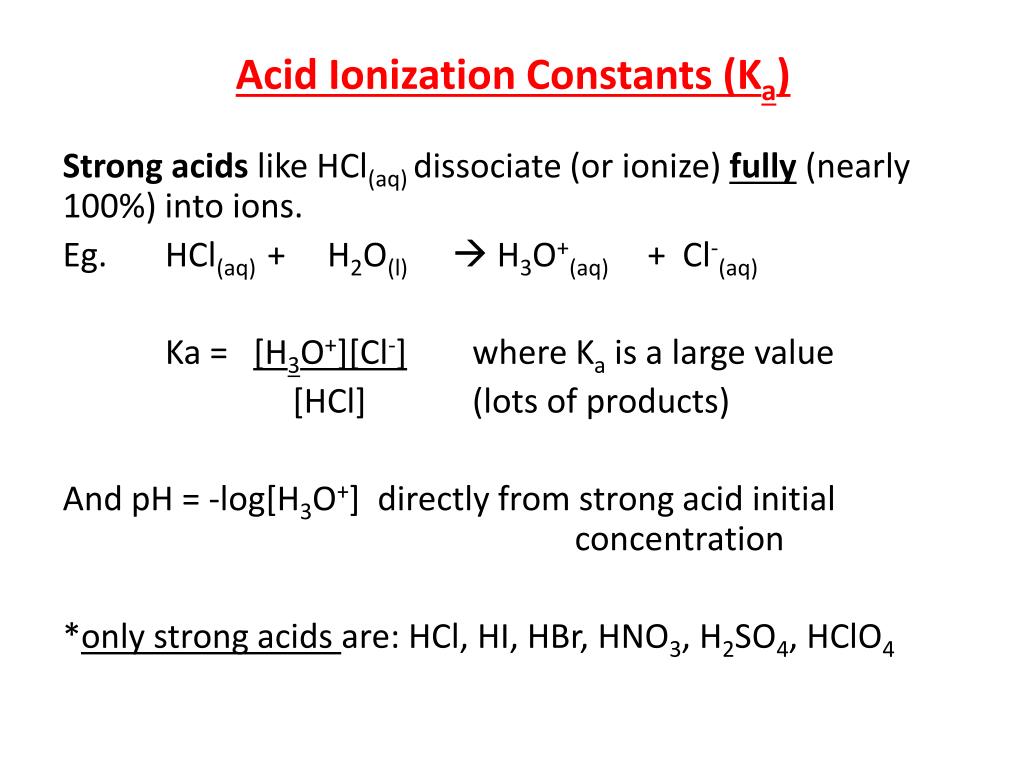
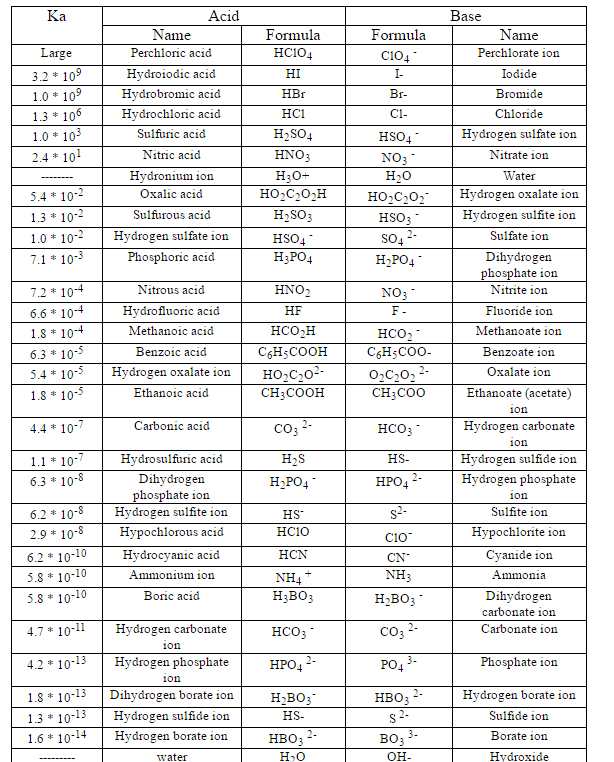
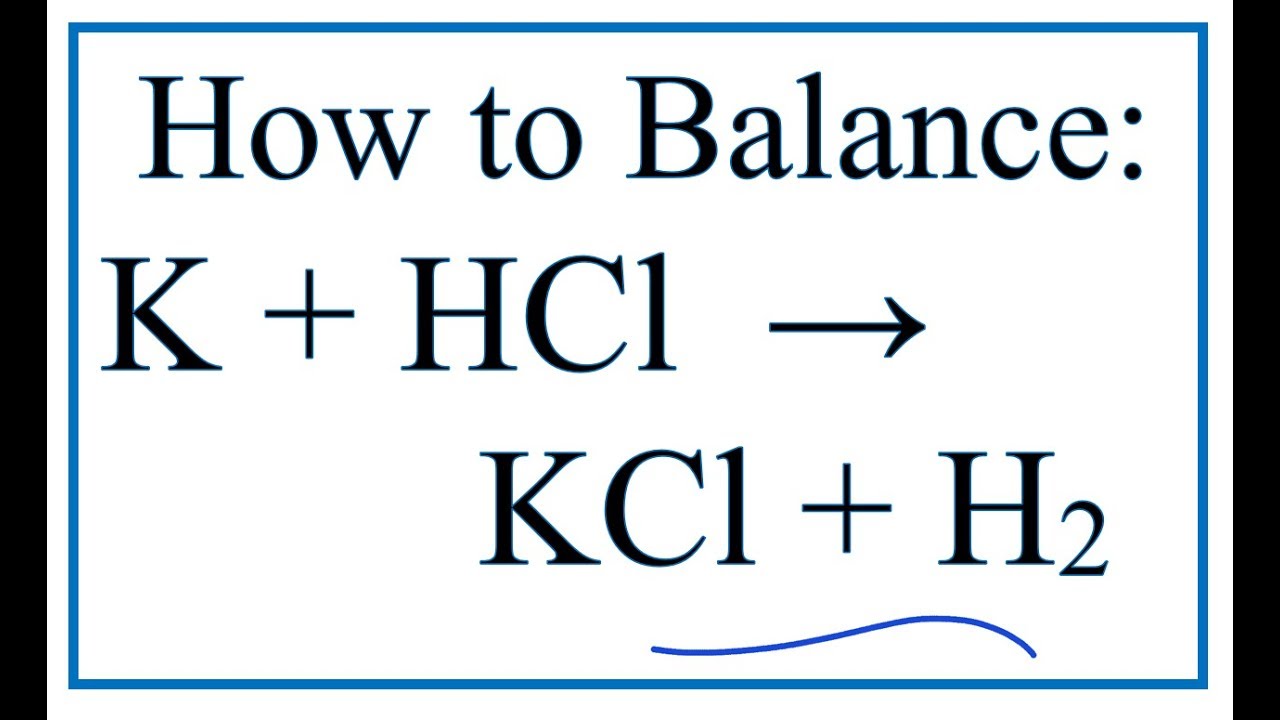
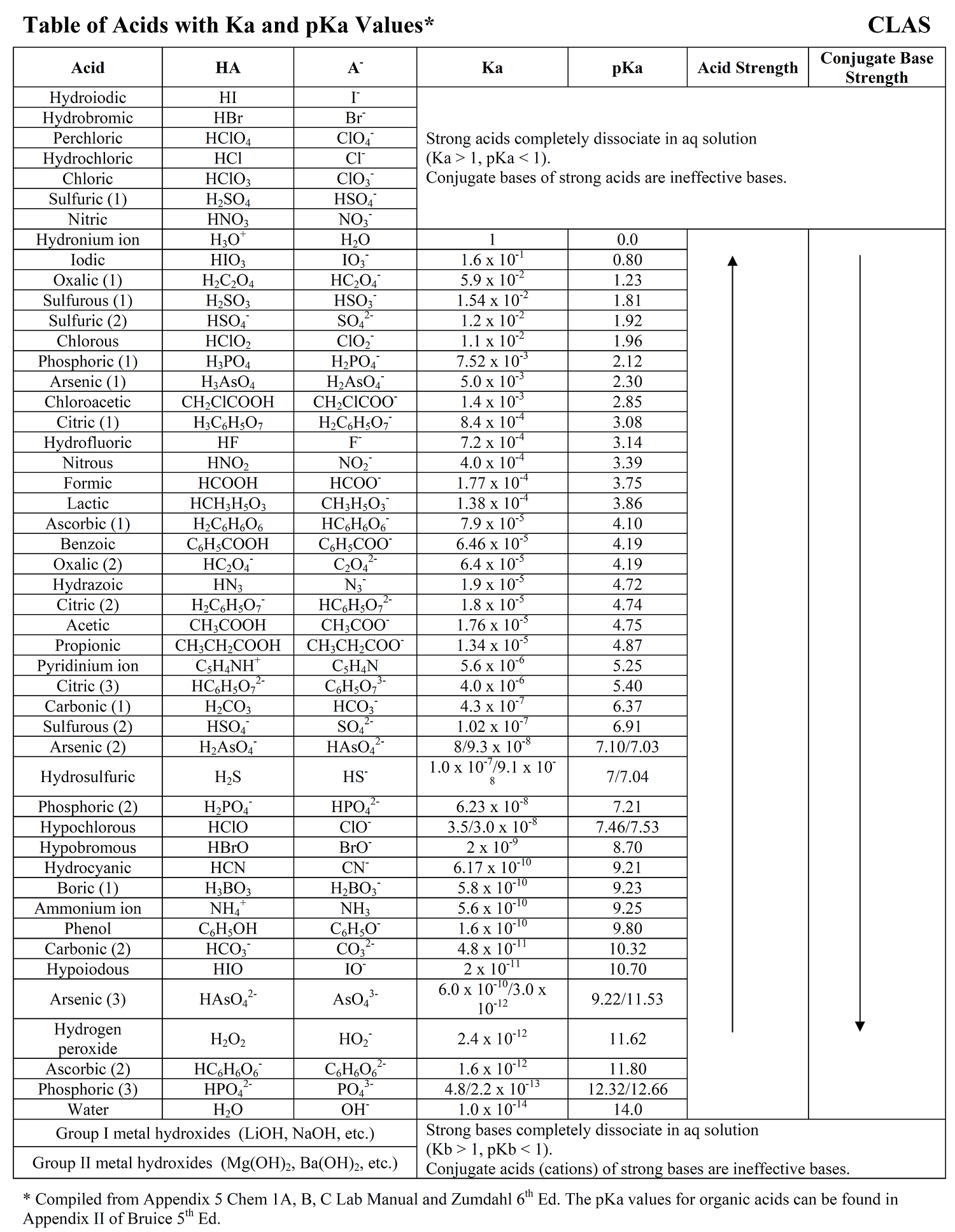
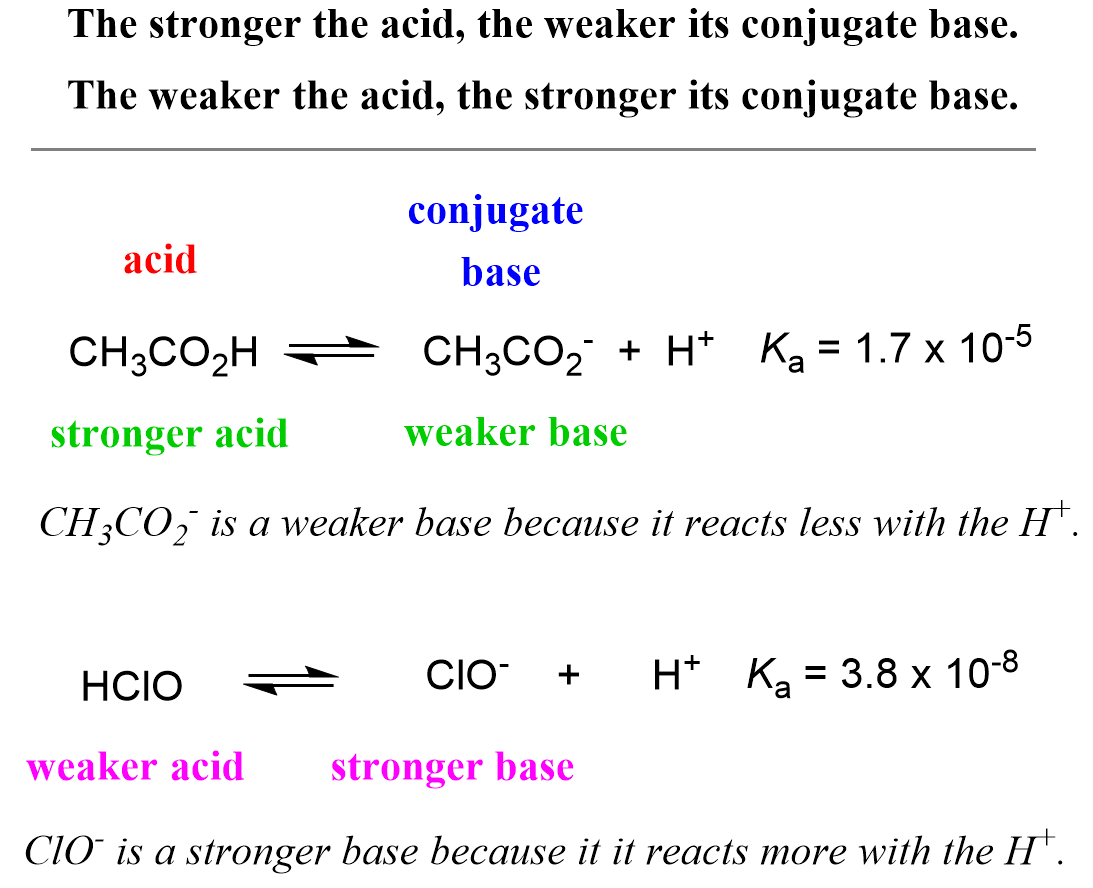
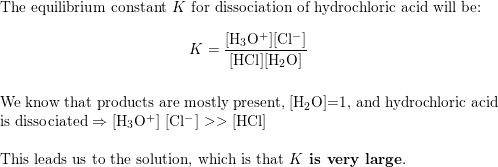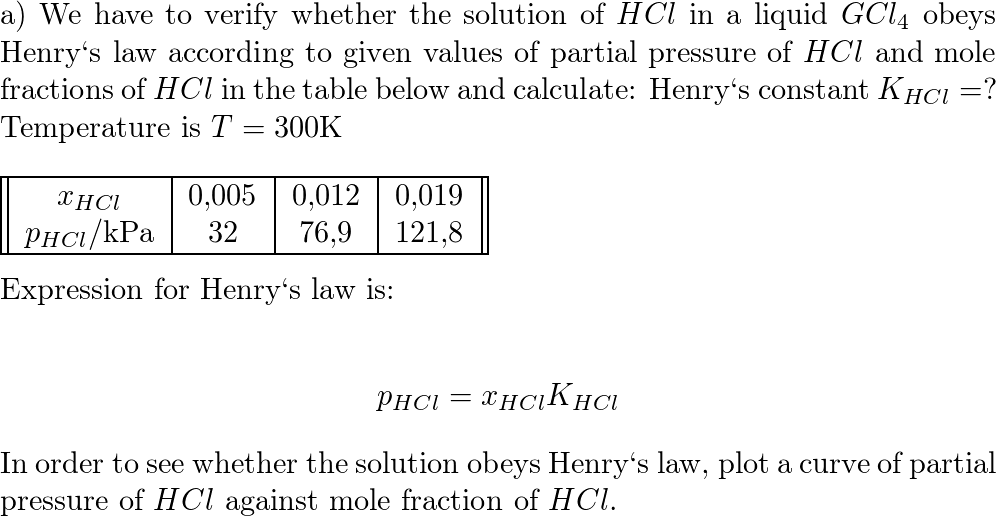Ka for CH3COOH is 1.8×10^-5. find out the % dissociation of 0.2M CH3COOH in 0.1M HCl solution? - EduRev NEET Question

The heat of formaiton of HCl at 348 K from the given data will be :1/2 H 2 g +1/2 Cl 2 g → HCl g H 2980= 22060 cal mol 1The
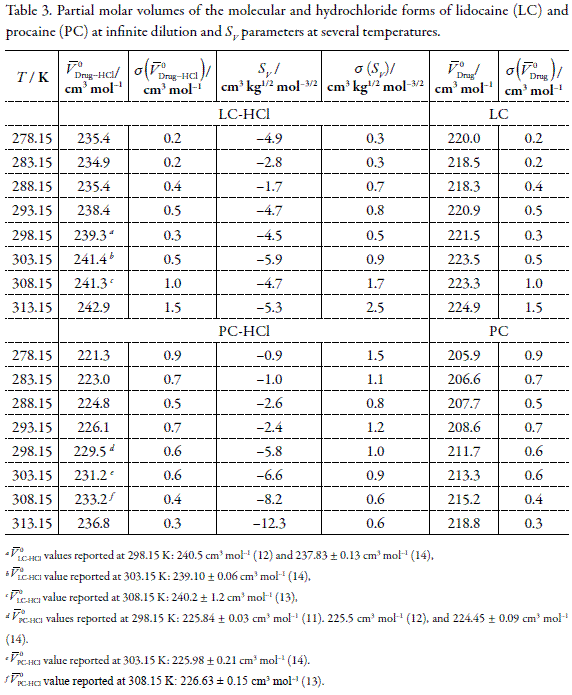
Apparent molar volumes of the anesthetic drugs procaine-HCl and lidocaine- HCl in water at temperatures from 278.15 to 313.15 K
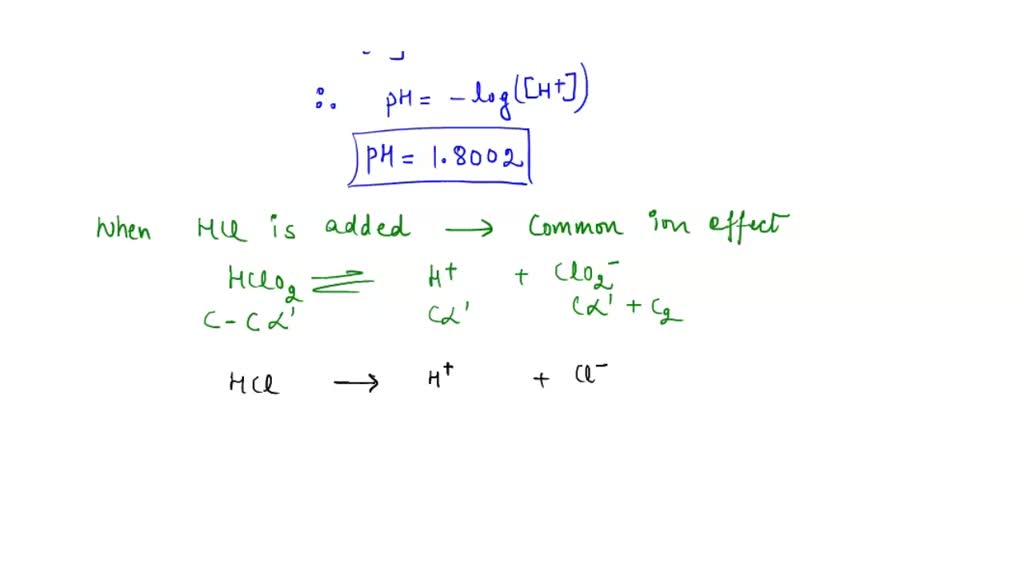
SOLVED: Determine the pH of a solution that is 0.00424 M HCl and 0.0228 M HClO2. The Ka of HClO2 is 1.1×10âˆ'2.

Reaction Kinetics of HCl Catalytic Oxidation over a Supported Cu-Based Composite Industrial Catalyst | Industrial & Engineering Chemistry Research

Lecture 1: Introduction and review –Quiz 1 –Website: –Review of acid/base chemistry –Universal features of. - ppt download